38 drug warning labels examples
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA warns about serious heart ... [06-07-2016] The U.S. Food and Drug Administration (FDA) is warning that taking higher than recommended doses of the common over-the-counter (OTC) and prescription diarrhea medicine loperamide ... › drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA cautions about using ... Testosterone replacement therapy is only approved for men who have low levels of testosterone related to certain medical conditions. Examples of these conditions include genetic problems, and ...
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication: Prescription Acetaminophen ... In addition, a Boxed Warning highlighting the potential for severe liver injury and a Warning highlighting the potential for allergic reactions (e.g., swelling of the face, mouth, and throat ...
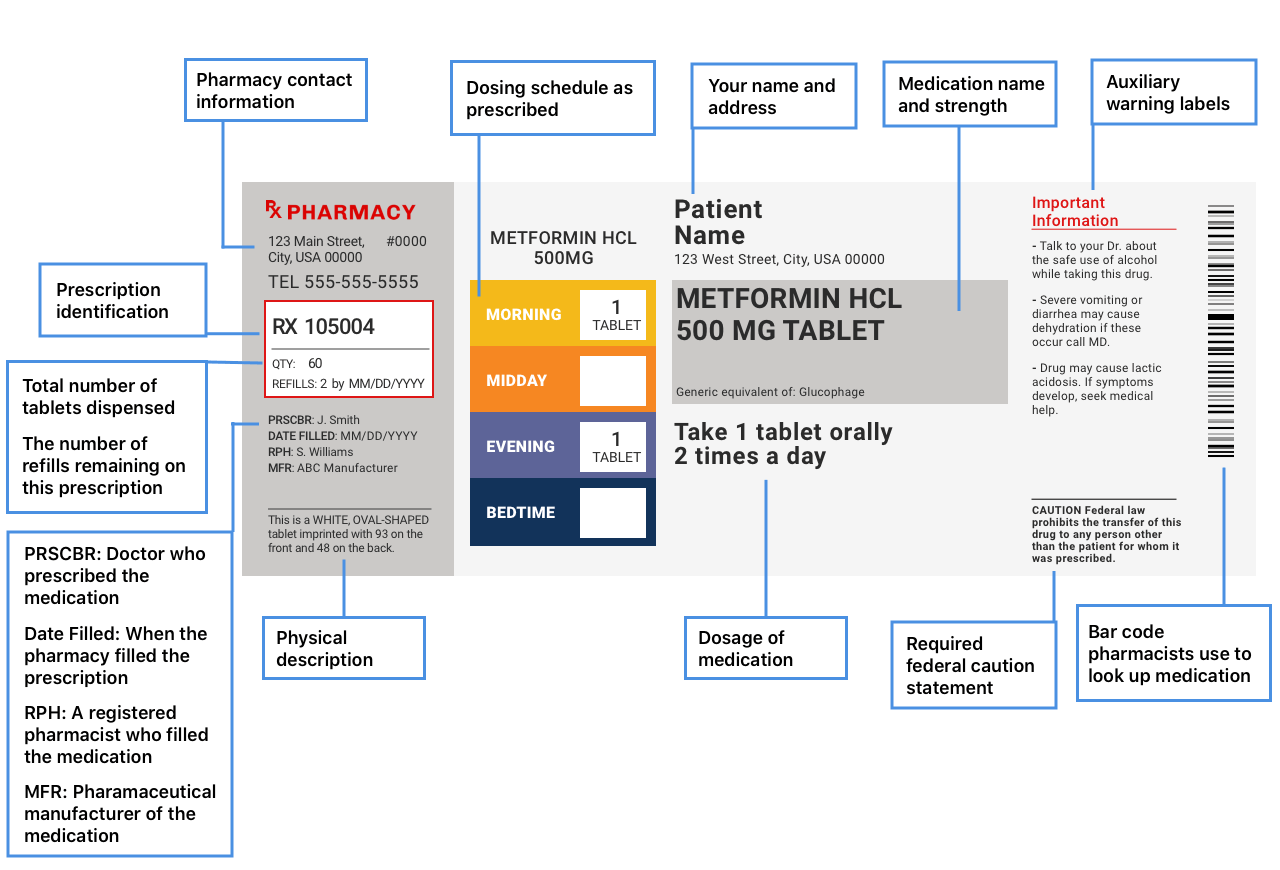
Drug warning labels examples
› food › food-labeling-nutritionChanges to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ... › tobacco-products › productsE-Cigarettes, Vapes, and other Electronic Nicotine Delivery ... Get an overview of FDA regulation of vapes, e-cigarettes, and other electronic nicotine delivery systems. You can also find statistics about current use. › drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA review results in new ... [ 12-14-2016 ] The U.S. Food and Drug Administration (FDA) is warning that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than ...
Drug warning labels examples. › drugs › drug-safety-and-availabilityFDA identifies sudden discontinuation of opioid pain medicines Examples of common opioids include codeine, fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, and oxymorphone. Health care professionals should not abruptly discontinue opioids in a ... › drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA review results in new ... [ 12-14-2016 ] The U.S. Food and Drug Administration (FDA) is warning that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than ... › tobacco-products › productsE-Cigarettes, Vapes, and other Electronic Nicotine Delivery ... Get an overview of FDA regulation of vapes, e-cigarettes, and other electronic nicotine delivery systems. You can also find statistics about current use. › food › food-labeling-nutritionChanges to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...

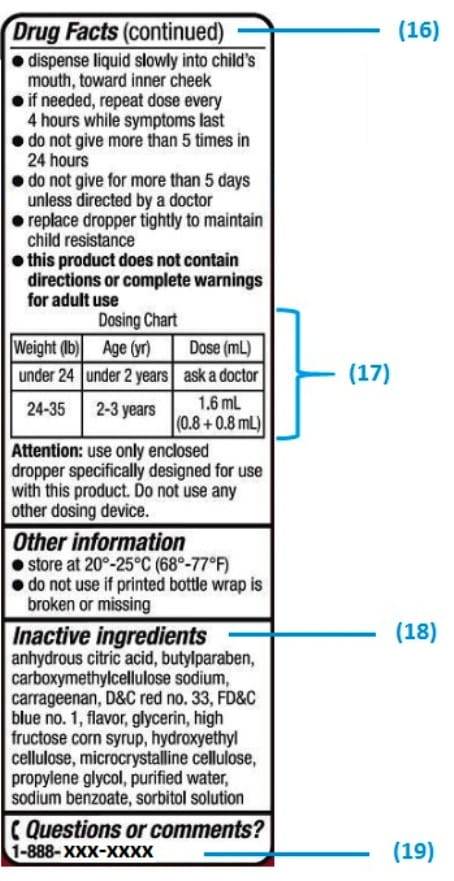

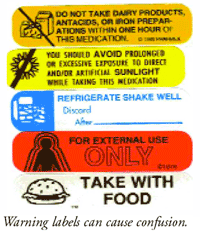

![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/3-Figure2-1.png)













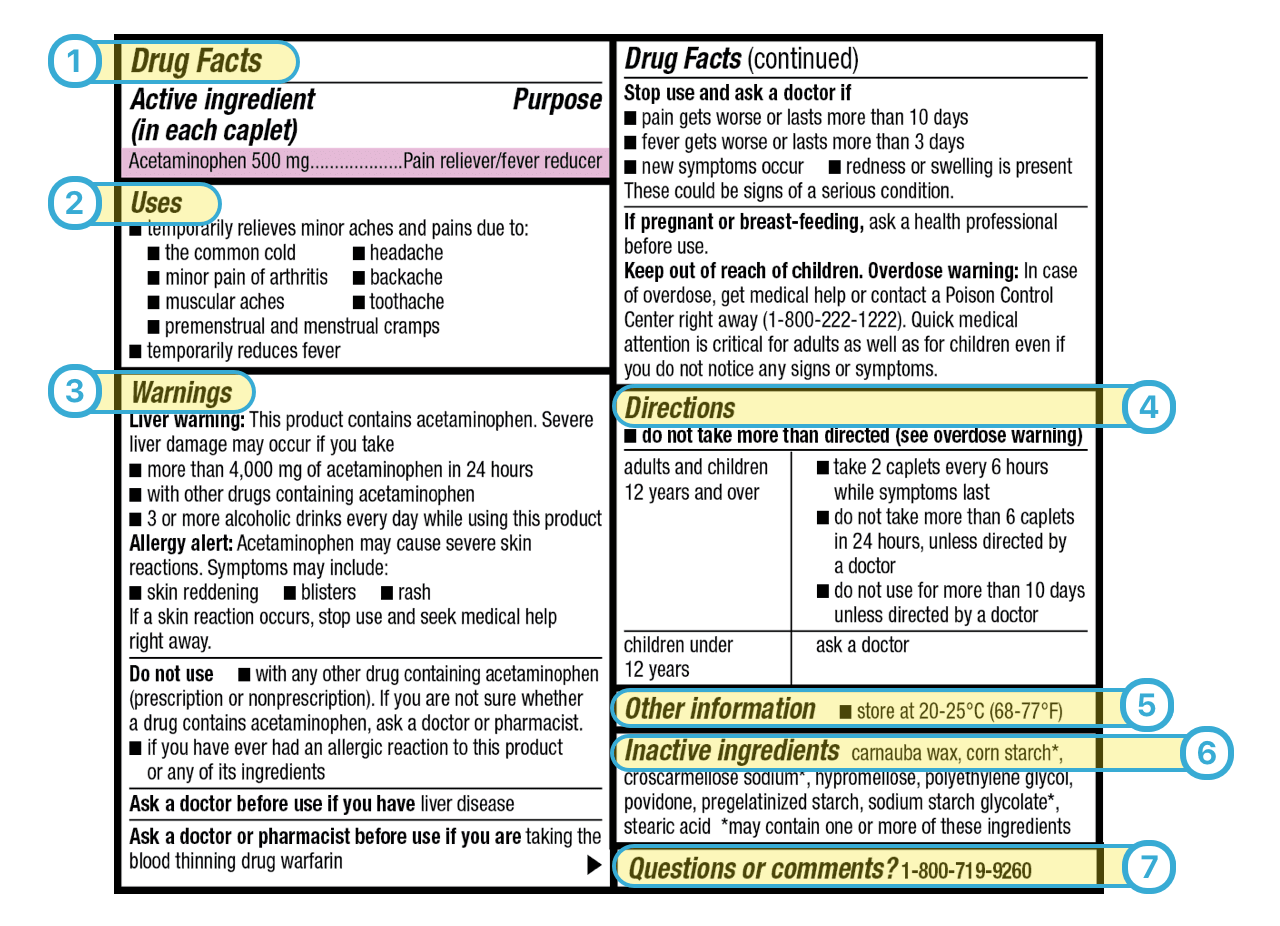



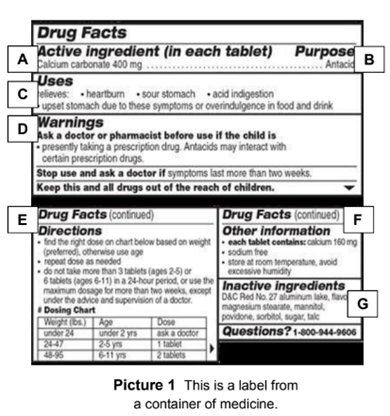
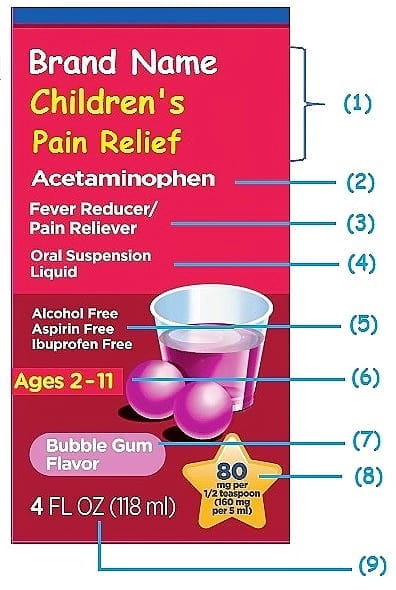
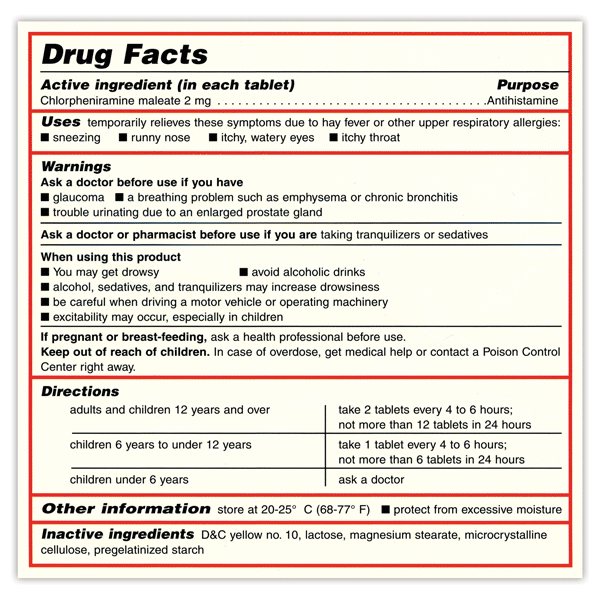


Post a Comment for "38 drug warning labels examples"